Department of Chemistry
Seoul National University
Our research lies in the interdisciplinary studies of inorganic chemistry, biochemistry, and protein engineering. We employ spectroscopic, kinetic, thermodynamic, and biochemical techniques/analysis to understand the orchestrated chemical reactions of metalloproteins and metalloenzymes.
Molecular design and evolution of artificial metalloenzymes
Merging inorganic cofactors with proteins can yield novel inorganic reactivities within protein space. Thus, we can design artificial metalloenzymes, explore the sequence-structure-function relationship, and simulate how metalloenzymes can emerge and evolve.

Chem. Sci., 2021
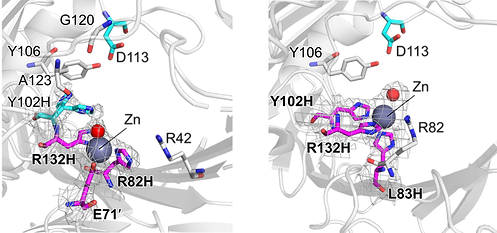
Nat. Commun., 2022

Inorg. Chem., 2021

J. Am. Chem. Soc., 2023

J. Am. Chem. Soc., 2023
Design of protein-protein interfaces for macromolecular self-assembly
Novel metal-coordination bonding or sequence modification can endow sufficient driving force for protein self-assembly. Then, we can create novel protein-based biomaterials or versatile protein scaffolds for enzyme design.

Biochemistry, 2021

Nat. Commun., 2019

Chem. Sci., 2024
Deciphering protein sequence-function relationship
We are also interested in elucidating protein sequence-function relationships. We perform bioinformatic sequence analysis and directed evolution to understand the consequences of sequence modifications in enzyme catalysis.

Sci. Rep., 2018

mSystems, 2021